© HENN
© HENN
Willkommen am CRTD
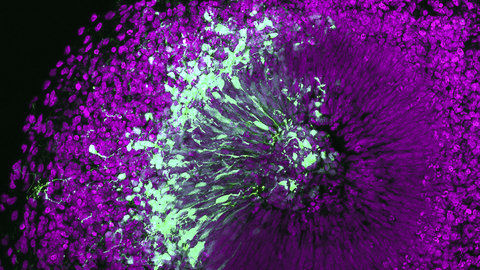
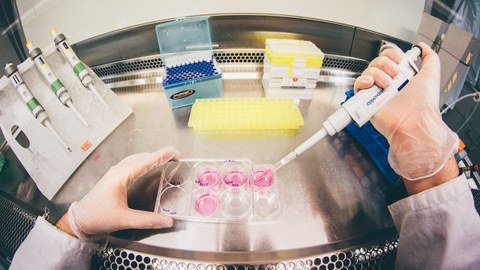
Die Mission des Zentrums für Regenerative Therapien Dresden (CRTD) ist es, die Biologie von Stammzellen und Geweben zu erforschen, um die Regenerationsprozesse von Organen zu verstehen. Daraus sollen neue Behandlungsmöglichkeiten für neurodegenerative Erkrankungen, wie Alzheimer und Parkinson, hämatologische Krankheiten wie Leukämie, Stoffwechselerkrankungen wie Diabetes, und Knochenerkrankungen entwickelt werden.
Unsere Partner
Folgen Sie uns!