Knopf Group - Biology of Bone Regeneration
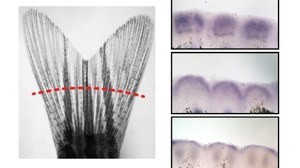
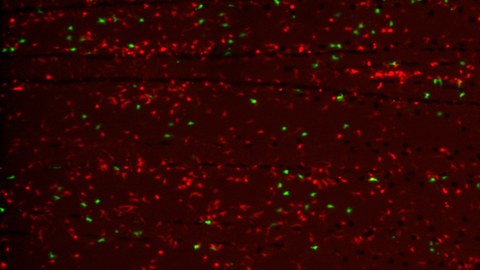
Bone fractures in humans heal well, especially at young age. Unfortunately, bone is not replaced after severe injury or upon long term treatment with certain drugs. In order to better understand mechanisms of successful versus impaired bone regeneration, we make use of highly regenerative zebrafish, and evaluate their bone forming capacity in different experimental contexts.
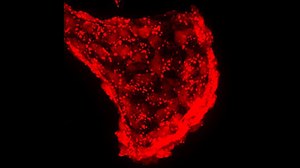 © Dr. Karina Geurtzen
© Dr. Karina Geurtzen
Our research
Our overall research goal is to unravel the mechanisms underlying bone formation with a view to exploit the regenerative capabilities of zebrafish. In particular, we investigate cellular and molecular mechanisms of succesful versus impaired bone regeneration in the zebrafish fin and skull, and take advantage of the zebrafish model to study the pathogenesis of different forms of bone fragility.